Abstract
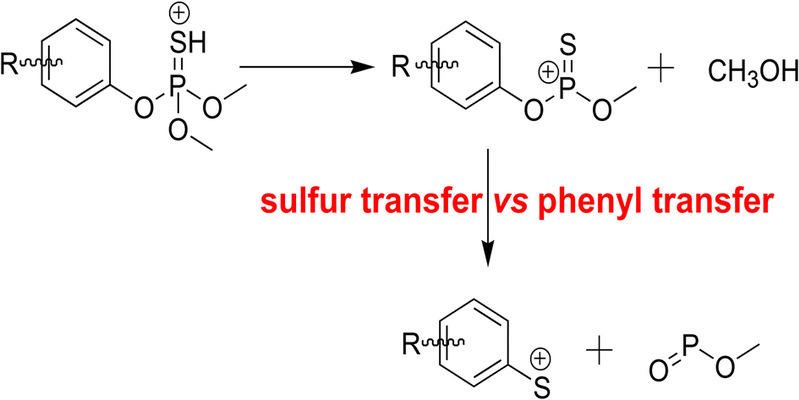
Collisional activation fragmentation of protonated phosphorothioates leads to skeletal rearrangement and formation of aryl sulfenylium cation (R-PhS+) via successive eliminations of CH3OH and CH3O–P=O. To better understand this unusual fragmentation reaction, isotope-labeling experiments and density functional theory (DFT) calculations were carried out to investigate two mechanistic pathways. In route 1, a direct intramolecular transfer of the R-phenyl group occurs from the oxygen atom to the sulfur atom on thiophosphoryl to form methoxylS-(3-methyl-4-methylsulfanyl-phenyl) phosphonium thiolate (a4), which subsequently dissociates to form the m/z169 cation. In route 2, the sulfur atom of the thiophosphoryl group undergoes two stepwise transfer (1,4-migration to theortho-carbon atom of the phenyl ring followed by 1,2-migration to theipso-carbon atom) to form an intermediate isomer, which undergoes the subsequent dissociation to form the m/z169 cation. DFT calculations suggested that route 2 was more favorable than route 1 from the point view of kinetics.
Graphical Abstract
 Sulfur Transfer Versus Phenyl Ring Transfer in the Gas Phase Sequential Loss of CH3OH and CH3O–P=O from Protonated Phosphorothioates.pdf
Sulfur Transfer Versus Phenyl Ring Transfer in the Gas Phase Sequential Loss of CH3OH and CH3O–P=O from Protonated Phosphorothioates.pdf